The coronavirus is crashing state and local budgets. Governments are dramatically increasing health care spending, even as tax revenue is tanking. Elected officials need to quickly find budget savings in order to continue providing necessary services, paying state and local government employees and providing health coverage.
Fortunately, there is a simple way to find some savings. State and local government leaders could save millions of taxpayer dollars were they more aggressive in encouraging the use of the generic versions of biologic medicines.
Generic prescription drugs account for 90 percent of the prescriptions sold in the United States.
Even though patients often prefer a generic because it’s much less expensive than its brand name counterpart, there hasn’t been a similar uptake of the generic versions of the rapidly expanding class of drugs known as biologics. That means patients and their health plans are spending more than they should.
Biologics are very complex drugs that contain living cells that are usually injected or infused into the body. Many of the new biologics target some of the most deadly and debilitating diseases such as cancer and arthritis.
Fortunately, we now have generic versions of several biologics, known as “biosimilars.” The number is small but growing—28 biosimilars have been FDA approved for nine brand name biologics.
Even though biosimilars cost less, they have yet to achieve the U.S. market penetration that traditional generics have. One reason for the slow uptake is the way prescription drugs are reimbursed.
When drug manufacturers release a new drug, they set its “list price,” though very few people actually pay that price. That’s because most Americans are in health plans in which pharmacy benefit managers (PBMs) negotiate both significant discounts off the original price and may also demand rebates from pharmaceutical companies for promoting a particular drug.
Because biosimilars cost less, the PBM middlemen often make more money by steering patients to the brand name product, which affects how much individuals, and especially employers who provide health coverage, pay for the drugs.
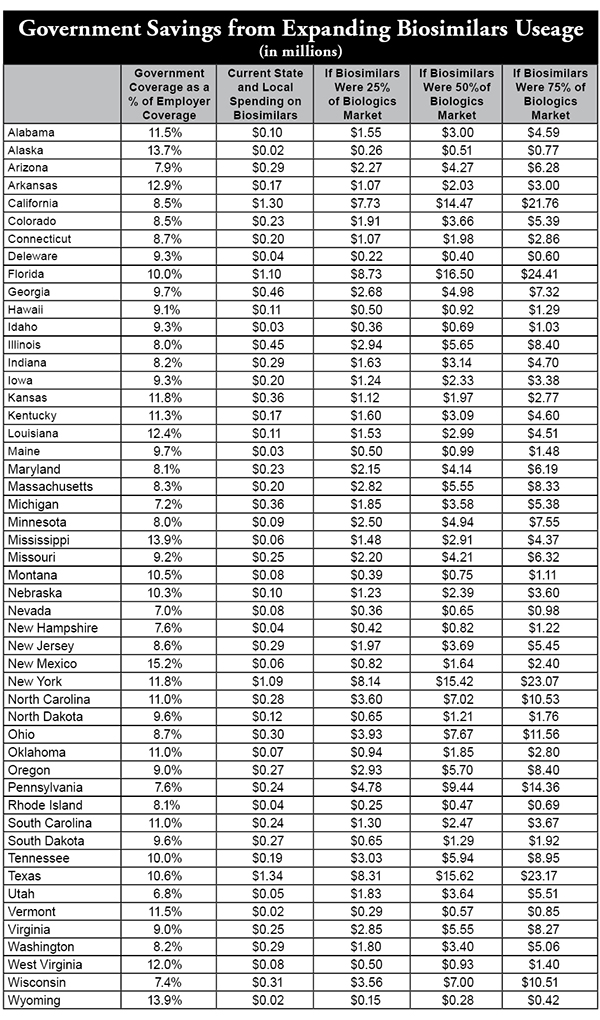
Wayne Winegarden of the Pacific Research Institute (PRI) recently published a paper entitled “The Biosimilar Opportunity: A State Breakdown,” which estimates cost savings if employers and state Medicaid programs substituted more biosimilars for brand name biologics.
The study estimates that nationwide, employers who provide health insurance currently save about $137 million annually by substituting biosimilars for brand name biologics. Were they to expand biosimilar use to 25 percent of the biologic prescriptions, the study estimated a savings nationally of $1.16 billion, $2.23 billion under a 50 percent substitution rate, and $3.33 billion if it were 75 percent.
Well, state and local governments provide health coverage to millions of full-time employees and make up a portion of PRI’s commercial coverage estimate.
In the table on the reverse side, we use Census Bureau data to identify the percentage of full-time workers who are state and local government employees in each state, the vast majority of whom will have qualified health coverage. Using Winegarden’s estimate for the money currently being saved by employers in each state, and the amount that could be saved were they to expand the use of biosimilars by 25, 50 and 75 percent, we provide an idea of how much each state could save.
And these savings could be only the beginning. Biosimilar competition tends to drive down the price of brand name biologics, and many more biosimilars are on the way, greatly expanding their potential for future savings.
Americans are the world’s top users of generics, but not of biosimilars. State and local governments should encourage a wider biosimilar uptake for their own employees as a way to provide quality care at lower costs—something state and local budgets need now more than ever.