Business for Affordable Medicine (BAM) is a coalition of 13 employers, 11 governors and a few local unions whose mission is “to close loopholes in the Hatch-Waxman Act that prevent timely access to less costly generic drugs after patents on brand drugs expire.”
However, BAM’s attempt “to close loopholes” is really the weakening of patent protections for the drug industry. Ironically, several of those BAM employers hold patents themselves, and some have aggressively defended their patents in court when they thought their patent was infringed.
The Issue for BAM Is Cost.
The real issue driving the BAM coalition is drug cost. Prescription drug utilization has been rising across all segments of the population for years—because there are more drugs to treat more illnesses. In addition, the higher prices of many newly patented drugs reflect the growing costs and time needed to create and develop a drug and get it to market. Health care costs are rising for several reasons, straining the bottom line. But instead of looking at the types of coverage they offer, BAM employers are looking for relief.
BAM Believes in Intellectual Property.
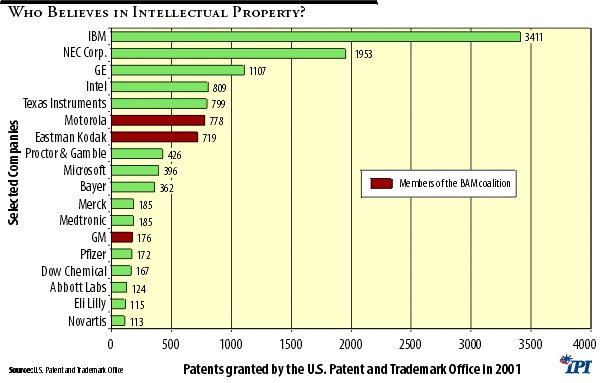
The U.S. Patent and Trademark Office (PTO) recently released its annual report identifying companies awarded patents in 2001. IBM was first on the list with 3,411 patents. But several BAM coalition members also made the list:
• Motorola was granted 778 patents and was ranked at 19;
• Kodak was ranked 20, with 719 patents;
• And GM, with 176 patents, was ranked 93.
BAM Members Defend Their Intellectual Property.
Patents play an important role in any company that deals with technology, as many BAM members do. And those members believe in defending their intellectual property. An Internet search revealed that several BAM coalition members are or have been plaintiffs in a number of patent lawsuits. For example:
• Motorola has filed 14 cases;
• Kodak has filed 11 cases; and
• General Motors is or was the plaintiff in 11 cases.
Drug Companies also Believe in Intellectual Property.
Patents are the lifeblood of the pharmaceutical industry, and a number of drug companies were on the PTO’s list. For example, Bayer topped the list for pharmaceutical manufacturers having been granted 362 patents in 2001, while AstraZeneca had the fewest on the list with 40 patents. Pfizer received 172 patents, fewer than GM. Merck had 185 and Eli Lilly 115.
The Life (and Death) of Drug Patents.
A patent for a new prescription drug lasts 20 years. (It was 17 years before the GATT agreement, which created an international standard for patent life.)
However, a drug manufacturer cannot sell its new drug until it has gone through clinical trials and been approved by the Food and Drug Administration (FDA). That research and approval process can take the first eight to 12 years of the 20-year patent life, leaving maybe 10 years or less of useful patent life in which the drug company that procured the patent and invested the research money has exclusive right to sell the drug—assuming the drug ever makes it to market.
Once a drug’s patent expires, the FDA permits generic companies to market an approved generic version that is the bioequivalent (i.e., the medicine has to be absorbed by the body at roughly the same rate) of a patented drug.
A System of Checks and Balances.
The right to profit from one’s creations and innovations is protected in the U.S. Constitution, which gives Congress the authority, “To promote the Progress of Science and useful Arts, by securing for limited Times to Authors and Inventors the exclusive Right to their respective Writings and Discoveries.” However, politics and practical concerns have generally determined how long the inventors and authors have exclusivity. For example, in contrast to patents, copyrights for books and other written material last for the life of the author, plus 70 years.
In 1984, the growing importance of pharmaceuticals in medical care and the desire to encourage more competition from the generic industry led to the passage of the Hatch-Waxman Act, which was meant to provide a system of checks and balances in the pharmaceutical industry.
It is one of those laws that, in retrospect, has worked very well. The “ innovator” drug companies that create new drugs retained enough incentive to continue to invest in researching and developing new drugs. Domestic R&D spending grew from $3.4 billion in 1985 to about $23.9 billion in 2001—a seven-fold increase.
But the generic industry has also prospered under Hatch-Waxman. Its share of the prescription drug market has grown from 19 percent of the volume in 1984 to 47 percent in 2000, according to IMS Health.
In addition, Hatch-Waxman created a relatively smooth and orderly process for adjudicating disagreements. Since 1984, 94 percent (or 7,781) of generic drug applications raised no patent issues. Of the 6 percent that did raise an issue over whether a generic company was infringing on a patented drug, few actually end up in court.
Congress should be very careful about changing the current system of checks and balances, as legislation introduced by Sens. Charles Schumer (D-NY) and John McCain (R-AZ) would do.
Brand Names vs. Generics.
The BAM coalition seems to operate under the assumption that a generic drug is the same or just as good as the brand name drug it seeks to replace. For example, BAM says “Changes to the Hatch-Waxman Act can improve competition and help employers reduce costs on some of the most popular medications.”
Ironically, GM does not draw that same conclusion when it refers to less-expensive imitation parts for GM cars and trucks, which GM calls “ counterfeit.” Here is what GM says:
• “ While imitation parts may be cheaper, they could cost you a lot more in the long run, and we’re not just talking about money.”
• “ For starters, imitation parts generally don’t last as long as genuine parts, making it necessary to replace them more frequently, costing you [the consumer] more money!”
• “ Worse yet, fake parts could cause major safety problems for you and your loved ones.”
• And finally, what might be called generic car parts “could ultimately cost you more of your hard-earned money, and that’s because legitimate companies such as GM are spending money to combat imitation parts producers, which can lead to higher overall parts prices.”
No pharmaceutical company could have said it any better than GM.
Conclusion.
Generic drugs play an extremely important role in health care, providing less-expensive alternatives to the brand name drugs. But they are not the innovator companies that create, research and market new drugs.
Innovator companies depend on the constitutional protections afforded intellectual property. Undermining those protections won’t increase competition and lower costs, it will increase costs and kill innovation. Just ask GM.
